UPNEEQ® (30 single-use containers)
UPNEEQ® (30 single-use containers)
Couldn't load pickup availability
ONLINE PURCHASE OF THIS PRODUCT IS ONLY AVAILABLE TO CURRENT PATIENTS OF OUR OFFICE. BY PURCHASING I CERTIFY THAT I AM A PATIENT AT THIS PRACTICE AND HAVE A CURRENT PRESCRIPTION ON FILE FOR THIS PRODUCT.
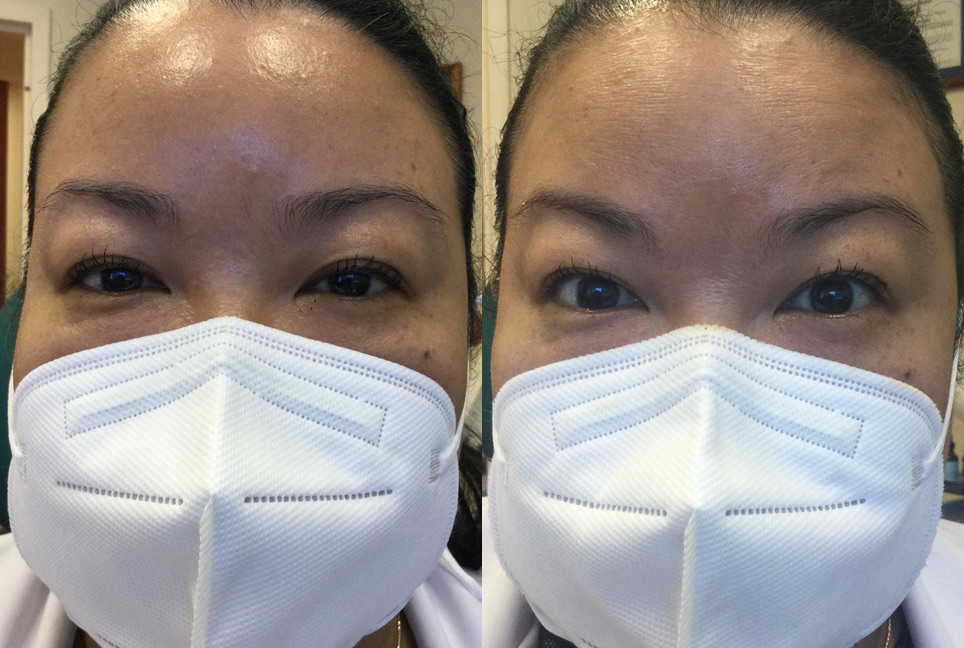
Imagine an EYE-OPENING Lift with a Daily Drop of Upneeq® (oxymetazoline hydrochloride ophthalmic solution), 0.1%. The only FDA-approved prescription eyedrop for acquired ptosis (low-lying lids) that lifts your upper eyelids to open your eyes.
This medication is used to treat a certain eyelid condition (acquired blepharoptosis) that causes drooping upper eyelid(s). It belongs to a class of drugs known as alpha agonists. Oxymetazoline works by making a certain eyelid muscle get shorter and tighter (contract).
Benefits
Benefits
- Lifts eyelid(s) quickly
- Most patients in clinical trials had a lift in their eyelids in as little as 2 hours
- 84% of patients had some form of improvement
- 74% of patients had more than a 50% improvement
- In one study, some patients saw a lift in their eyelids as fast as 5 minutes after the first dose
- Significantly improves upper field of vision
- In clinical trials, Upneeq helped patients with acquired ptosis see more–on the first day of treatment!
- 7.8% of patients had some form of improvement
- 40.8% of patients had at least a 50% improvement on Day 14 (2 hours after applying Upneeq)
Skin Type
Skin Type
Usage
Usage
Ingredients
Ingredients
Each mL of UPNEEQ® (oxymetazoline hydrochloride ophthalmic solution) 0.1% contains 1 mg of oxymetazoline hydrochloride, equivalent to 0.09 mg (0.09%) of oxymetazoline free base. The ophthalmic solution contains the following inactive ingredients: calcium chloride, hydrochloric acid (used to adjust pH to 5.8 to 6.8), hypromellose, magnesium chloride, potassium chloride, sodium acetate, sodium chloride, sodium citrate, and water for injection.
Share